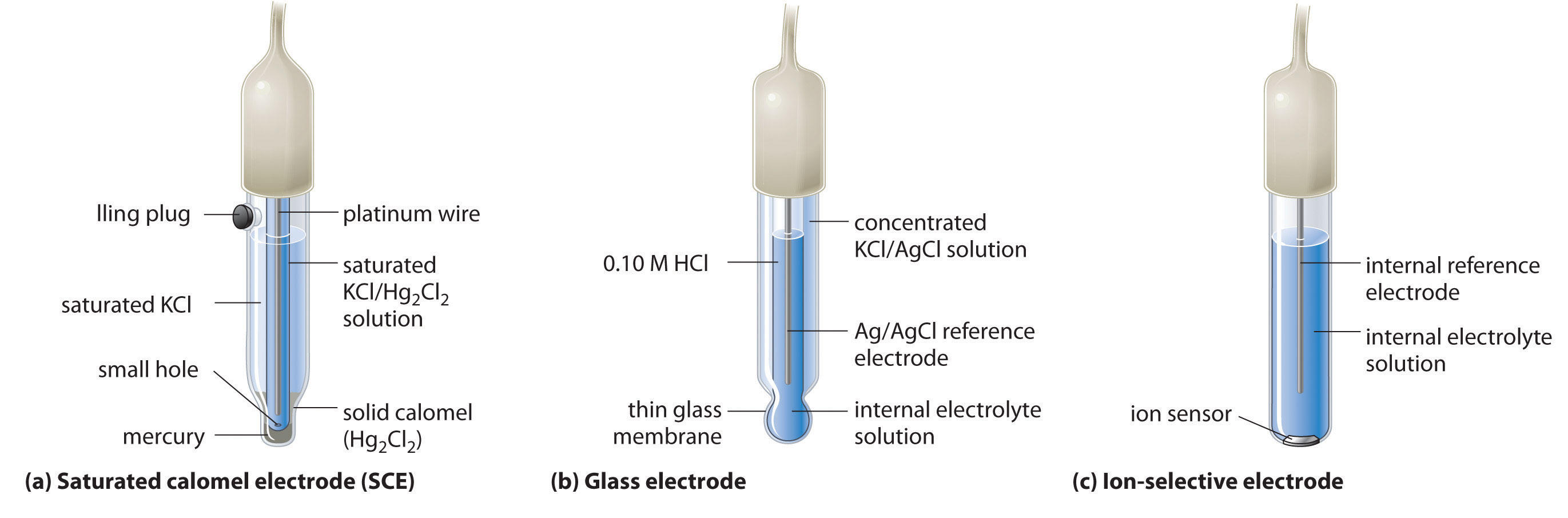
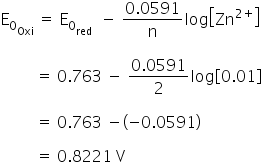Empowering Zn Electrode Current Capability Along Interfacial Stability by Optimizing Intrinsic Safe Organic Electrolytes - Ilyas - Angewandte Chemie International Edition - Wiley Online Library

EIS plots for zinc electrodes in 6 M KOH containing different amounts... | Download Scientific Diagram
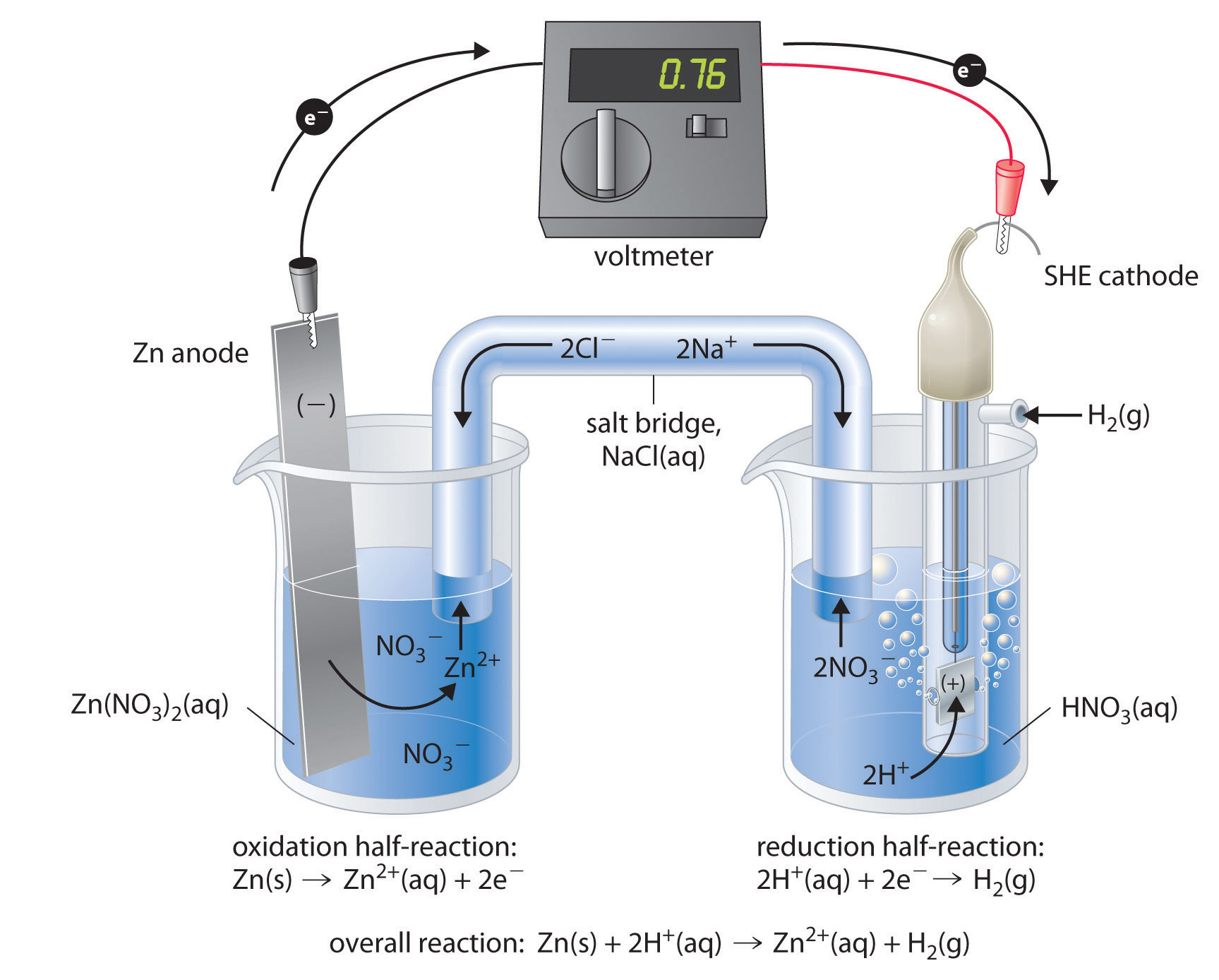
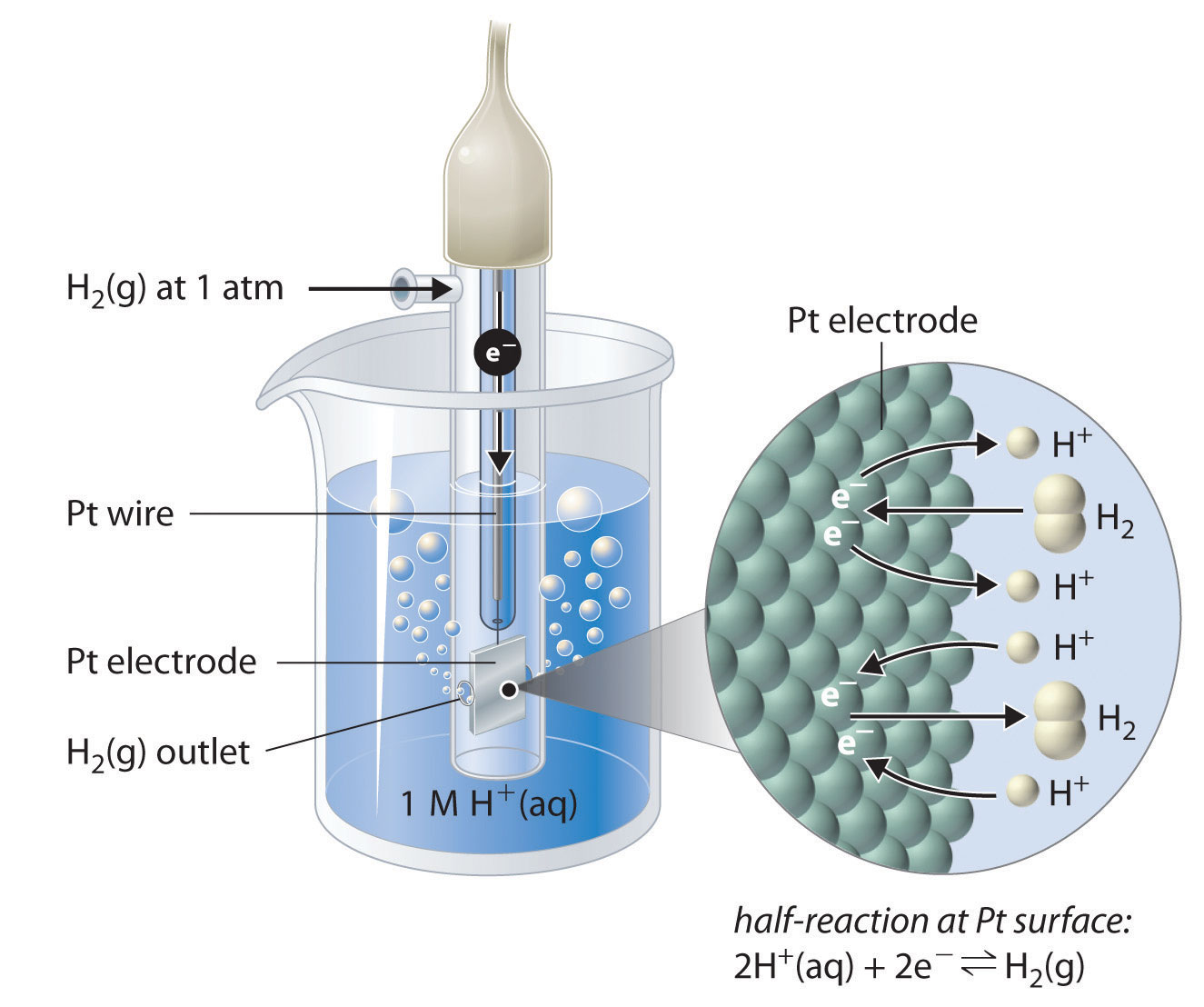
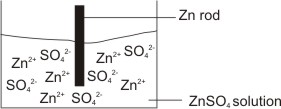
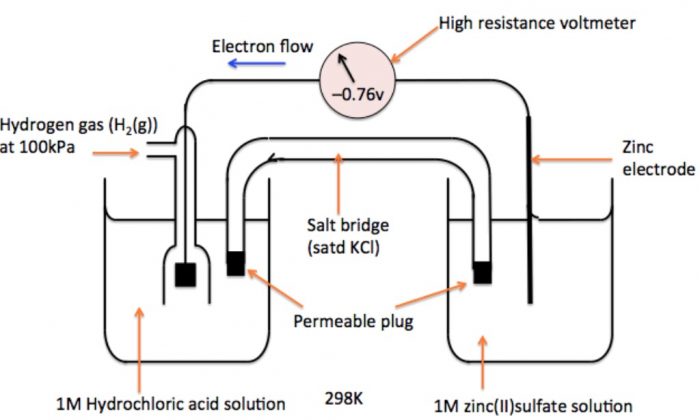
How would you determine the reduction potential of Zn/Zn^2+ (aq)? - Sarthaks eConnect | Largest Online Education Community

The standard reduction potentials of zinc, cadmium and copper are - 0.76 V, - 0.4 V and 0.34 V respectively. Select the correct statement.

Standard electrode potentials for the cathode and anode reactions in... | Download Scientific Diagram

The standard reduction potentials of zinc, cadmium and copper are - 0.76 V, - 0.4 V and 0.34 V respectively. Select the correct statement.
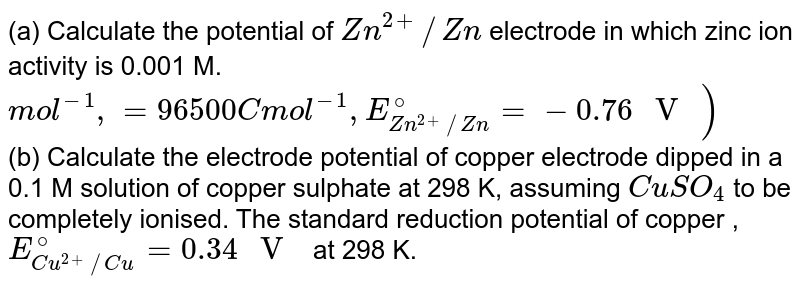
a) Calculate the potential of Zn^(2+)//Zn electrode in which zinc ion activity is 0.001 M. mol^(-1),=96500 C mol^(-1), E(Zn^(2+)//Zn)^(@)=-0.76" V ") (b) Calculate the electrode potential of copper electrode dipped in a